I simply recently gave a chat at an event Salters Hall in central London. Over the buffet lunch, I fell into dialog with a senior researcher from a world chemical agency, an pure chemist. Reminiscing about vanished practices, I requested him whether or not or not he remembered anyone ever making hooch inside the lab. He recalled {{that a}} scholar in a single different lab had carried out this. Was he being coy, I questioned?

It was as quickly as common for researchers to make batches of festive alcohol. The preps involved steeping fruit in alcohol and together with spices, sooner than final distillation. The glassware was always saved strictly separate to steer clear of contamination, and all the recipes began with the instruction to start out out from each 96% or spectroscopic grade alcohol, nevertheless on no account absolute. The reason is all the best way right down to a discovery made in Bristol merely as distillation was being reworked into an accurate science.
The scientist in question was Sydney Youthful, born near Widnes in Lancashire. His well-off dad and mother despatched him to the Royal Institution College, just about the one secondary school in Liverpool that gave an education to ‘the sons of respectable people’. Youthful would go on to Owens College in Manchester the place he studied with Henry Roscoe and Carl Schorlemmer who had been specialists in inorganic and pure chemistry respectively.
In 1881 he spent a time interval in Strassburg inside the lab of the pure chemist Rudolf Fittig – the place he grew to change into a gifted glassblower – and on returning to Britain studied in Bristol with thought-about one in every of Fittig’s former faculty college students, the Scottish chemist William Ramsay. Youthful turned decisively within the route of bodily chemistry, getting right down to put thermodynamics to the check out. With Ramsay, Youthful confirmed that ammonia could be decomposed over iron filings, and that the response was an equilibrium, an assertion that proved a key milestone inside the enchancment of the Haber course of.
He then turned to the difficulty of sublimation. The Clausius–Clapeyron relation linked the temperature and the vapour stress of liquids and neatly outlined why that that they had ‘boiling components’, nevertheless did the an identical apply to sublimation? Starting with ice, Youthful confirmed that there was fairly clear relation between the temperature of ice deposited on a thermometer in vacuum, and an utilized stress. Pure compounds like benzene and camphor fitted the pattern too, serving to to draw traces on stress–temperature part diagrams. Over the subsequent decade Youthful and Ramsay would map out the thermodynamics of fairly just a few packages exploring vapour pressures, essential phenomena, and studying the impression of chemical dissociation.
When Ramsay left Bristol in 1887 to succeed Alexander Williamson at Faculty College, London, Youthful took his place. By then the two had become company buddies they normally continued to publish collectively until the Nineties when Ramsay discovered the noble gases.
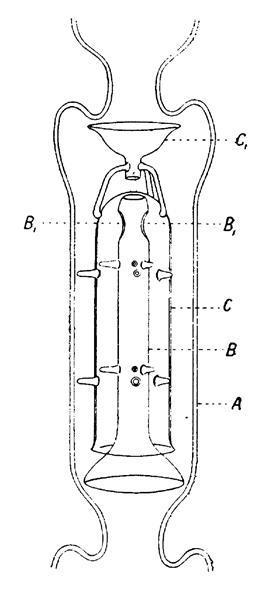
Youthful’s curiosity in thermodynamics meant that he wished very pure starting provides. For liquids, distillation was the one recreation in town, nevertheless the quite a few designs of distillation glassware all had limitations. Wurtz’s bulbs had been solely fairly atmosphere pleasant whereas the bubbling ‘dephlegmators’ with platinum gauzes like Linnemann’s baskets, and Lebel–Henninger’s fractionator held up an extreme quantity of liquid, inflicting wastage. With a scholar, G Thomas, he developed a fractionating column by means of which a group of indentations inside the prolonged tube (prefiguring Vigreux’s later design) held gauzes each pierced by a U-tube to allow lots of the liquid to empty down the centre of the tube.
To reduce the hold-up extra, Youthful devised a rod with plenty of flat disks alongside its measurement that stood on a tripod of prongs, and which could be inserted into a normal nonetheless head. The outcomes had been markedly increased, and this led him to an extra enchancment: an ‘evaporator’ nonetheless head, the place the liquid was pressured to adjust to alternating upward and downward paths by a group of central tubes capped by inverted test-tubes, oddly reminiscent of a multistage diffusion pump. Curiously, Youthful on no account mentions ground area in any of his papers, which was the essential factor to the operation of these columns. A gorgeous set of them is on present at Trinity College Dublin the place Youthful would end his career.
As he developed these stills, Youthful labored on the distillation of varied mixtures with Emily Fortey, a chemist who would later push for the Chemical Society to admit girls. The pair found that together with benzene to moist alcohol led to a three-component azeotrope that could be distilled away to depart absolute ethanol, though traces of benzene remained that had been onerous to eradicate. For a few years this was the principal industrial course of for producing absolute ethanol, until molecular sieves provided an alternate, and was the reason for that substance’s proscription from these lab hooch recipes.
Making moonshine inside the lab is unthinkable proper this second. Nonetheless inside the archives at UCL, William Ramsay’s dinner e book accommodates an entry for an evening in November 1889 when Ramsay hosted Dimitrii Mendeleev. Among the many many guests was Sydney Youthful. I’m optimistic that there have been a group of toasts proposed on the end of the dinner. What had been they ingesting? Was it Scotch? Vodka? Or was it some Ramsay and Youthful homebrew?
Acknowledgments
A lot of buddies recalled hooch recipes and procedures. Ben Vitality despatched me pictures from Dublin.
