The character of an ‘unidentified product’ in consuming water disinfected with chloramines, which serves over 113 million of us throughout the US alone, has lastly been revealed by researchers throughout the US and Switzerland. First detected practically 40 years up to now, the molecular ion, known as chloronitramide, has in no way been characterised sooner than, so its toxicology is unknown. Nonetheless, its focus in some samples of faucet water taken in areas akin to California and Texas and its similarity to harmful molecules warrant investigation, the researchers warn. The invention might also add to earlier issues in regards to the utilization of chloramines to sterilise faucet water.
Chlorine, a low-cost and very environment friendly disinfectant, is basically probably the most extensively used chemical for steralising consuming water the world over. Nevertheless throughout the Seventies, scientists found that it might react with pure matter to offer harmful chemical compounds akin to trihalomethanes and haloacetic acids. The US Environmental Protention Firm (EPA) subsequently regulated the utmost permissible concentrations of various of these in faucet water. To chop again the manufacturing of these disinfection by-products, many water remedy authorities throughout the US and one other nations along with the UK have partially or fully switched to disinfecting water with chloramines, which can be usual by combining chlorine with ammonia.
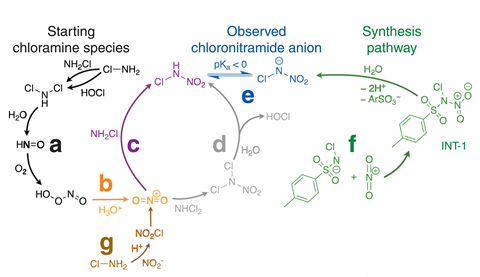
Throughout the early Nineteen Eighties the signal of a mysterious molecule was observed in UV absorbance spectra of chloraminated water. Environmental engineer Richard Valentine on the School of Iowa, US, and his colleagues carried out intensive investigations throughout the Nineteen Eighties and Nineties, displaying the circumstances beneath which it usual. Nonetheless, the molecule’s precise development remained unclear.
‘We confirmed that there was a critical product usual when monochloramine decayed – which it does naturally, and it wanted to be in pretty extreme focus,’ explains Valentine. ‘What made it robust is that it had the an identical ultraviolet spectra as a result of the reactant monochloramine.’ He compares looking for the development as ‘like looking for a needle in a haystack the place the entire gadgets of hay are needles’.
Throughout the new evaluation, environmental engineers led by Julian Fairey on the School of Arkansas, US, collaborated with analytical chemists at ETH Zurich led by Juliana Laszakovits. The researchers used various utilized sciences to which Valentine’s group had not had entry, akin to ion chromatography coupled to electrospray ionisation–mass spectrometry. ‘This mixture of strategies isn’t so typically utilized in environmental analysis, nevertheless it certainly’s truly good at separating anions,’ says Laszakovits.
The molecule was too small for its development to be determined by its mass spectrometry fragmentation pattern. Nevertheless mass spectra of isotopically labelled samples allowed the researchers to confirm that it contained two oxygen atoms, two nitrogen atoms and a chlorine atom. 15 N NMR and infrared spectroscopy then helped to confirm its development.
The researchers’ experiments suggest that the compound principally varieties in chloraminated consuming water by hydrolysis of dichloramine to nitroxyl, which reacts with dissolved oxygen to offer peroxynitrite. ‘One among many decomposition merchandise of peroxynitrite is a nitrating agent known as the nitronium cation, and that’s the linchpin that then reacts with each monochloramine or dichloramine to offer this compound,’ says Fairey.
As a result of the molecule has in no way been isolated sooner than, its toxicity is unknown. Nonetheless, the researchers used an EPA algorithm known as Generalized Be taught All through to match its structural and chemical similarities to recognized molecules. They concluded extra analysis is urgently warranted, notably provided that concentrations in just a few of their samples are bigger than these of regulated hazardous compounds. In precise reality, Fairey initially began the enterprise studying how nitrosamines – a class of compounds which is perhaps considered ‘doable carcinogens’ – sort in chloraminated water. ‘In the event you occur to eradicated one in all many oxygen atoms from the nitro group, you’d have a nitrosamine,’ says Kristopher McNeill at ETH Zurich in Switzerland – the place chloramination of water is illegal.
‘I consider it’s great work by the exact of us to find out it out’, says Valentine. ‘I’ve been prepared 40 years for someone to pick out up on my work.’
Environmental engineer Daniel McCurry of the School of Southern California, US, says that the discovering is ‘very very important’ and believes that it should instant a re-evaluation of the utilization of chloramine as a water disinfectant. He notes totally different disadvantages akin to a better propensity to corrode lead pipes and ugly ‘pool scent’. ‘Any plant throughout the US could sufficiently take care of their water with chlorine with out violating disinfectant byproduct tips within the occasion that they’ve been ready to and had the sources in order so as to add certain totally different remedy steps like activated carbon or membranes.’ he says. ‘They’re possibly going to have to do that anyway on account of PFAS legal guidelines. So hopefully that is prone to be a two birds, one stone state of affairs.’
Correction: This textual content was updated on 25 November 2024. An earlier mannequin incorrectly stated that the chloronitramide anion contained only one nitrogen atom.
