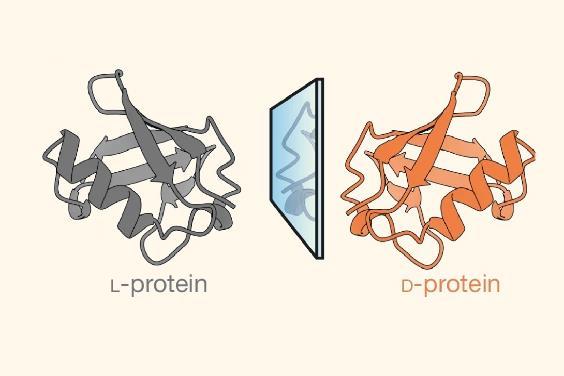
The delicate chiral buildings of proteins typically suggest they’re going to solely work along with ligands with a complementary chirality. Now, researchers have seen that disorganised proteins and peptides have equal interactions with strings of L- and D-amino acids, which could have implications in drug discovery and help to uncover the causes of chirality on early Earth. ‘It is attainable for a [disordered] protein to work along with its ligand regardless of its chirality,’ explains co-first author Estella Newcombe from the School of Copenhagen, Denmark.
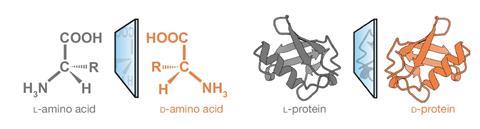
Nearly every pure protein consists of concatenations of L-amino acids. As quickly as completely folded, the three-dimensional development is stereospecific to a sequence of molecules, often known as ligands, normally left-handed throughout the case of small peptides. As a result of this truth it was ‘assumed {{that a}} native L-protein [couldn’t] work along with its D-peptide ligand’, says Newcombe. ‘We confirmed that L- and D-proteins work collectively, if such interaction stays significantly [disordered and] dynamic.’ Nonetheless, as a result of the protein development turned additional ordered, the opportunity of recognising the mirror substrate decreased.
Nonetheless, an rising number of disordered peptides and proteins have been present in latest occasions. ‘It’s increasingly more apparent {{that a}} very important proportion of the proteome is disordered,’ explains Jane Clarke, an expert on protein folding at Wolfson College on the School of Cambridge, UK. No matter earlier assumptions, some disorganised proteins ‘variety specific and tight binding complexes’, although they’re surprisingly ‘“blind” to specific structural alerts’, she gives. ‘In some methods, that’s baffling.’
On this study, researchers chosen pairs of proteins and ligands, by which definitely one among them stays disordered throughout the unbound state. By synthesising every L- and D-peptides and proteins, they could study the compatibility in increasingly more organised buildings, and measure the binding energy, along with the sensitivity to chirality. ‘The additional ordered [the] development … the a lot much less chance {{that a}} protein may work along with its mirror ligand,’ says Newcombe.
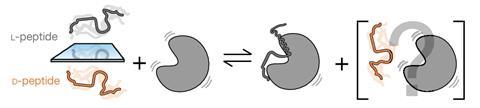
It’s a ‘distinctive means’ to examine the stereospecificity of disorganised peptides and proteins, explains Clarke. Researchers made a ‘truly clever number of examine packages, [supported by] the facility of a variety of devices to seek out out the structural and dynamic properties of proteins and complexes’.
‘There is a presumption that protein–protein interactions are stereospecific, nonetheless this displays that [some] interactions don’t appear to depend on chirality,’ says Michael Kay, an expert in mirror-image biology on the School of Utah, US. Whereas fully-folded proteins have solely a small diploma of flexibility, ‘disordered proteins undertake a numerous array of buildings, additional akin to strands of spaghetti’, gives Kay, or as one commenter dubbed them ‘floppy fuzzballs’. Proper right here, if ‘every components of a flowery [are] disordered, the heterochiral interaction [is] equal to the pure homochiral interaction’. In homochiral interactions, the two gadgets at play have the equivalent handedness.
The implications of the invention transcend structural biology. This may advance the ‘design of drugs to give attention to disordered proteins, central to many signalling processes throughout the cell, and thus central to understanding [several] sickness packages’, explains Clarke. On account of our our our bodies broadly metabolise L-peptides and proteins, understanding the subtleties of structural recognition of mirror molecules may convey creative choices for centered treatments. ‘D-peptides are invisible to degradation, thus additional regular,’ gives Newcombe. Finally, D-peptides and proteins may current ‘a model new avenue for specializing in sicknesses attributable to disordered proteins, [such as] neurodegenerative sicknesses’, she gives.
Moreover, ‘the authors intriguingly speculate that the heterochiral interactions seen may need existed in a prebiotic world, sooner than homochirality was established’, says Kay. Such suppositions, alongside additional analysis, may uncover clues within the path of clarifying key components in molecular evolution, along with the dominance of left-handed amino acids, peptides and proteins. Up to now, it’s confirmed the prospect that protein–protein interactions existed on prebiotic Earth. However it raises questions too. Some proof implies that ‘every L- and D-amino acids had been present sooner than life started on Earth, nonetheless within the current day … D-[residues] have grow to be very unusual’, says Newcombe. ‘Our study displays L- and D-[peptides and proteins] work collectively in certain conditions, so it truly raises additional questions: why did we discover your self with one enantiomer and by no means the other?’