They’re saying seeing is believing nevertheless our understanding of chemical strategies predominantly depends on second-hand observations and measurements. A shade change can suggest {{that a}} response has occurred, NMR peaks current how atoms in a development are linked and enantiomeric additional measurements level out the proportion of assorted enantiomers. Nonetheless, chemists don’t usually see the molecules themselves.
X-rays and electrons
Crystallography is doubtless one of many few exceptions, turning these secondary measurements proper right into a conclusive 3D development. Single crystal x-ray diffraction – basically probably the most extensively used of these strategies – has been recognized for better than 100 years and is the gold commonplace of development identification all through every chemistry and biology. When x-rays are fired at a crystal sample, its widespread inside development scatters gentle systematically and, by recording this diffraction pattern and dealing backwards, it’s doable to calculate the 3D development of the molecule.
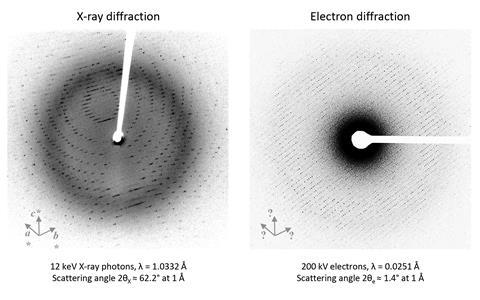
This structural notion is vital in fields like precision drug docking and enzyme engineering the place spatial information performs a central place. Nonetheless, as x-rays work collectively comparatively poorly with matter, the method requires big crystal samples, normally between 20–200μm, meaning the overwhelming majority of small molecule and protein samples are unsuitable for analysis.
Electrons, nevertheless, work along with matter spherical 1000 cases additional strongly than x-rays, making it doable to analyse crystals solely a billionth of the dimensions. The caveat: on account of sample damage solely a single image may be collected from each crystal and deciphering the following diffraction patterns is way tougher.
All through detection, plenty of the small print concerning the diffracted waves is misplaced and a complete bunch of explicit particular person datasets collected from fully totally different crystal samples ought to as a consequence of this reality be combined manually to infer the missing particulars and assemble a coherent development. Within the meantime, the bigger affinity of electrons for matter will improve the number of secondary and tertiary collisions – a phenomenon usually often called dynamical scattering – meaning the connection between diffracted beam depth and molecular development turns into blurred. Consequently, electron diffraction (ED) experiments are every troublesome and time-consuming, with samples restricted to two-dimensional crystals to steer clear of dynamical scattering.
MicroED is born
Membrane proteins are considerably troublesome to crystallise and it was the frustration born of this awkward heart ground between x-ray’s impractically big crystals and ED’s 2D slices which led structural biologist Tamir Gonen to begin to combine these methods.
In 2004, his crew used low-intensity ED to resolve the development of double-layered aquaporin crystals. Nonetheless pretty than combining images from fully totally different crystal samples, they calculated once more from the diffraction pattern of a single sample using a method employed in x-ray diffraction to recuperate the waves’ missing information. The two-layer samples have been technically 3D crystals, moreover casting doubt on the assertion that dynamical scattering limits electron diffraction to single-layer crystals. ‘The question then was, why weren’t totally different of us able to treatment 3D constructions?’ says Gonen. ‘I started pondering maybe the limitation was how they collected and listed the data.’
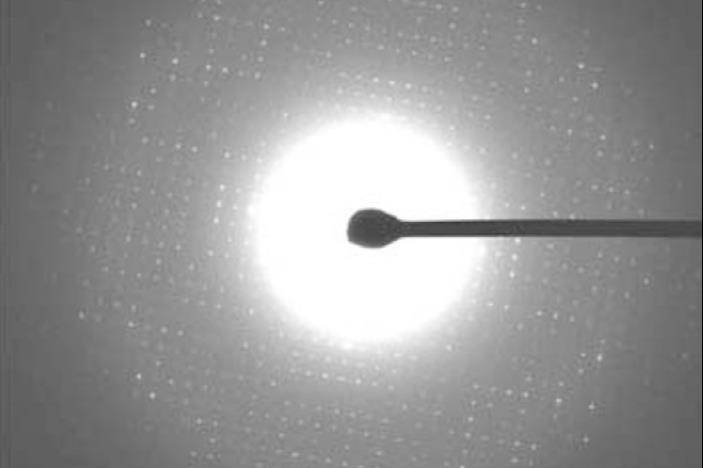
Indexing is doubtless one of many ranges in diffraction pattern analysis and entails determining the vectors (or axes) for each of the peaks inside the diffraction information. It’s a comparatively easy course of for x-ray experiments as a result of the three axes are sometimes obvious from the curved patterns. Nonetheless electron diffraction produces flat line-like patterns, making it unattainable to reliably decide the third vector.
‘I realised that that’s because of the experiment itself. The wavelength of x-rays is pretty big so the scattering angle can be very big and that’s why you probably can parse out the vectors,’ explains Gonen. ‘In electron diffraction, the wavelength is way shorter and that signifies that the scattering angle can be very small. So although we now have a 3D crystal, mainly we’re taking a two-dimensional slice.’ Nonetheless, if plenty of 2D slices may be taken from a single crystal sample, each image would then be mathematically related, making it doable to look out the third vector and determine the whole 3D development.
This notion was the catalyst for Gonen to develop a hybrid method usually often called microED. Microcrystal samples spherical 50nm are mounted onto a rotatable stage in a transition electron microscope (TEM) set to diffraction mode. The stage is then rotated as a low-intensity electron beam is fired on the sample, with a fast digital digital camera recording the diffraction. ‘The know-how that we developed in my group is that this regular rotation of the stage. Electron microscopes shouldn’t designed for movement so we constructed controllers to coordinate the movement of the stage with the digital digital camera recording,’ says Gonen.
Each video physique is then processed with tailor-made x-ray software program program to generate a high-resolution 3D development. That’s one different key advantage of the method in response to Gonen. X-ray purposes are terribly well-established and minor modifications, supported by practically every provider, permit present software program program to interpret electron diffraction patterns, along with x-rays.
Fixing protein points
The crew debuted this new method in a proof-of-concept analysis in 2013, fixing the development of the recognized protein lysozyme and immediately attracting the curiosity of the structural biology group.
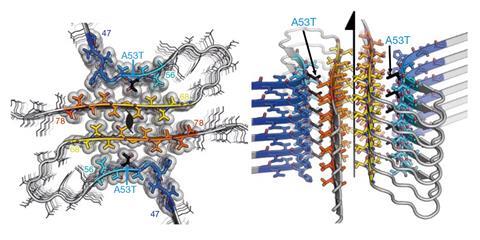
For David Eisenberg, a protein biologist specialising in neurodegenerative sickness, microED proved the reply to a 10-year-old disadvantage. Eisenberg’s crew have been engaged on the development of Lewy our our bodies, protein aggregates implicated in neuronal damage in Parkinson’s sickness, nevertheless the toxic core unit usually often called α-synuclein proved considerably troublesome to crystallise. ‘The crystals have been so small, merely 100nm, that there was no answer to control them or put them in an x-ray beam,’ says Eisenberg.
Nonetheless, these tiny crystals have been the best dimension for microED. After seeing Gonen’s 2013 paper, Eisenberg reached out and, inside per week, the elusive development of α-synuclein had been solved, marking the first unknown protein development determined by this method.
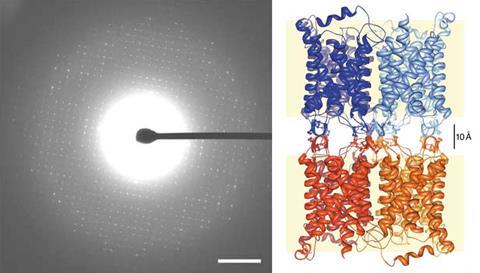
Throughout the years since, microED has been used to resolve varied totally different tough protein constructions nevertheless there are nonetheless plenty of wise factors to deal with sooner than the method turns into mainstream all through biology. Particularly, the sophisticated sample preparation step requires a extreme stage of technical expertise.
Proteins naturally carry water inside their development. Nonetheless the electron microscope ought to operate in a vacuum to steer clear of background scattering by air, meaning the experimental circumstances hazard dehydrating the protein and collapsing its development. ‘We’ve been freezing the crystals – when it’s in a thin layer of ice, that protects it from the vacuum,’ says Gonen. ‘Nonetheless you then come into this powerful area the place, what’s among the finest methods to freeze your crystal? Too thick and the electrons will probably be unable to penetrate. Too skinny and likewise chances are you’ll crush your crystal.’
Non-biological samples are additional easy to rearrange and Gonen believes this method may be a really useful addition to the small molecule characterisation toolbox.
Probing purity and polymorphs
Small molecules are sometimes easier to crystallise than proteins, nevertheless the bulk nonetheless in no way sort crystals of sufficient dimension for conclusive x-ray analysis. MicroED as a consequence of this reality offers the potential to drastically improve the scope of molecules acceptable for structural analysis, giving chemists entry to a complete bunch additional information elements with comparatively little effort. ‘With microED, we’ll obtain constructions for spherical 95% of small molecules,’ says Gonen. ‘These constructions are normally very high-resolution, throughout the 1Å mark, so we’ll see explicit particular person atoms and know precisely what the hydrogen bonding networks are. The form of information can then be fed into machine finding out and precision drug docking.’
Crucially, this method moreover detects crystal varieties (polymorphs) present inside a single pure sample, one factor which has terribly obligatory implications for the pharmaceutical enterprise. Drug polymorphs can have very fully totally different bodily properties which immediately impact the bioavailability and, consequently, the efficacy and safety of the last word drug product. The distinct kinds of an vigorous compound are moreover eligible for separate patent security so firms have an monetary curiosity in determining polymorphs at an early stage. The extreme choice and small crystal dimension makes microED a fragile and quick totally different to x-rays which many inside the enterprise are beginning to uncover.
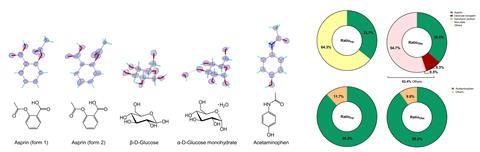
Nonetheless previous determining mixtures of crystal constructions, Gonen’s crew has currently written software program program enabling them to analyse product mixtures. The model new code collects microED information from each crystal in a sample, analysing the datasets individually and providing an estimation of the mass of explicit particular person crystals. ‘We’ll combine this to do pretty thorough compositional analysis. In case you had a mixture of an unknown, chances are you’ll determine what’s in it and the way in which a variety of each one with none prior information,’ says Gonen. The group demonstrated this new performance on pharmacy samples of aspirin and paracetamol with the last word analysis fastidiously matching the composition listed by the producer.
Entry denied
Nonetheless whatever the manifold advantages of this method for the chemistry group, microED continues to be practically unparalleled amongst small molecule chemists. ‘Entry continues to be an infinite disadvantage,’ concedes Gonen. The expensive and specialist instruments – the TEM – combined with the technical information required to take microED measurements means the method is almost impenetrable to those not already expert in crystallographic methods. ‘We’ve written scripts that let of us to rearrange the microscope and accumulate the data routinely, but it surely absolutely’s the extreme stage of expertise required for the instruments that’s the limitation,’ says Gonen.
This entry disadvantage is the vital factor subject stopping researchers in numerous areas exploring the potential of the method, says supramolecular chemist Bernd Schmidt. Supramolecular chemistry occupies a careless heart ground the place characterisation is frightened. The large symmetric constructions give deceptively straightforward NMR spectra, whereas the weak bonds can lead to sophisticated fragmentation by mass spectrometry. X-ray crystallography might be one of the best method nevertheless the innate properties of supramolecular compounds limit the efficacy of this method.
‘Coping with and rising supramolecular single crystals could also be very tough. Our compounds are intrinsically not regular on account of they’re dynamic covalent compounds,’ says Schmidt. ‘Now we’ve these giant molecules with giant cells the place solvent or associates are ordered or disordered all through the pores which intrinsically leads to poor diffraction at lower angles and poor-resolution images.’
Schmidt believes microED would provide an ideal decision to this dilemma. The small crystal dimension and quick acquisition cases could dramatically streamline workflows, allowing researchers not solely to additional efficiently characterise final provides, nevertheless to know the topology and geometry of samples at an earlier stage and possibly even scan response mixtures to hint the self-assembly course of.
Nonetheless, at present, he can solely speculate how the method could enhance his evaluation. ‘The TEM machines which may be on the market at universities are completely used already for various capabilities, so there’s very restricted availability to fiddle. The entry barrier moreover in regards to the information and software program program could also be very extreme,’ he says. ‘I really feel there’s an infinite explosion about to happen when anybody fuses this altogether into one factor accessible and user-friendly.’
Automating for accessibility
These missed alternate options are exactly what impressed analytical instrumentation agency Rigaku to launch the XtaLAB Synergy-ED in 2021. ‘We observed [microED] as an up-and-coming method that will probably be useful to the an identical group of scientists that we in the intervening time promote x-ray instruments to,’ explains Joe Ferrara, chief scientific officer at Rigaku. ‘Our model was that with just a few minutes of teaching, someone would have the power to walk as a lot because the XtaLAB Synergy-ED and accumulate information instantly.’

In distinction to the tailor-made TEMs utilized by academics like Gonen and Eisenberg, the XtaLAB Synergy-ED is especially designed for microED experiments, with automated information assortment, image processing and analysis, making the method accessible to a complete crystallography novice. ‘It’s taken microED from a evaluation endeavor and developed it proper right into a enterprise system,’ says Mark Benson, head of single crystal enterprise at Rigaku. ‘The potential market is massive, nevertheless just a few scientists are acutely aware of how electron diffraction could help them.’
Consciousness is the vital factor subject driving demand, says Ferrara, with early curiosity coming largely from the MOF/COF group who already make use of electron diffraction. Throughout the years since, Rigaku have obtained inquiries the pharmaceutical and battery industries and further space of curiosity capabilities equivalent to sample analysis for museum collections.
The issue now, says Benson, is convincing grant reviewers of microED’s potential and guaranteeing the required funding is in place to permit departments and corporations to invest on this know-how. New machines begin at spherical €1.5 million (£1.3 million), roughly equal to a mid-range NMR machine, and last yr Rigaku supplied two machines to the world’s first devoted nationwide microED service on the UK’s Nationwide Electron Diffraction Facility in Southampton.
Lastly, the hope is that microED could flip right into a routine part of chemical analysis, pretty than a specialist method. ‘In 5 to 10 years the imaginative and prescient might be that every fundamental school can have one amongst these units,’ says Ferrara.