We now have Jöns Jacob Berzelius to thank for allotropes. He first coined the time interval throughout the early nineteenth century to counsel that two forms of the an identical ingredient (or compounds with comparable compositions) might very properly be completely totally different, resembling monoclinic and rhombic sulfur. At situations, the thought has been mocked. Victorian economist William Stanley Jevons, as an illustration, wrote in his 1874 e-book Guidelines of Science ‘Every sulphur and selenium may be thrown into a lot of curious states, which chemists conveniently eradicate by calling them allotropic, a time interval freely used once they’re puzzled to know what has occurred’. However it has moreover been re-examined as our chemical understanding has superior and the Worldwide Union of Pure and Utilized Chemistry (Iupac) defines allotropes as merely ‘completely totally different structural modifications of a part’.
Some elements have many allotropes. None further so than carbon whose sp1, sp2 and sp3 hybridised bonding decisions suggest it might properly exist in an infinite number of preparations. As Andy Extance explains in our fourth twentieth anniversary article, discoveries surrounding the buildings and properties of fullerenes, carbon nanotubes and graphene ignited a rush of research into new forms of pure carbon. When Chemistry World was publishing its very first factors, an explosion of latest carbon allotropes and distinctive nanocarbons was merely over the horizon. The most recent carbon allotropes to be realised embrace graphullerene – which the workforce who made it dubbed graphene’s superatomic cousin – and a sequence of cyclocarbons.
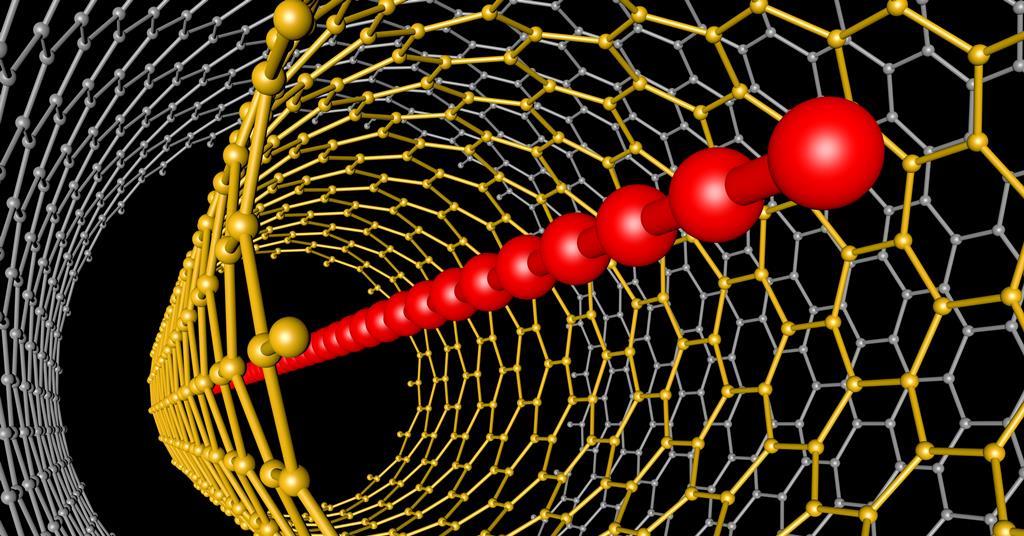
One carbon allotrope that seems to be significantly elusive is a sequence of sp-hybridised carbon atoms typically known as carbyne. Synthesising this linear building in a well-defined form is proving to be every robust and dangerous. Theoretical fashions counsel carbyne may be loads stiffer than diamond, have a band gap that is delicate to pressure and be able to change from a semiconducting to a metallic state. Such big incentives have impressed synthetic chemists to get creative, as an illustration, by way of the usage of multiwalled carbon nanotubes as nanoreactors. Nevertheless undisputed proof that carbyne can exist as a pure bulk supplies stays to be lacking.
One different topic surrounding carbyne is whether or not or not it counts as an allotrope the least bit. Marc Monthioux, writing in Carbon Developments,1 says no – allotropes should be able to assemble into periodic buildings. So cyclocarbons, fullerenes and carbon nanotubes additionally must be stripped of the title.
The logic behind Monthioux’s argument is reasonably priced nevertheless given chemists – to not level out Wikipedia and totally different public sources of information – use the time interval allotrope pretty loosely, I imagine it’s unlikely to attain loads traction.