Scanning transmission electron microscopy experiments have uncovered the structural changes a ruthenium catalyst undergoes that enhance its train all through an ammonia cracking response. The findings might help researchers design increased heterogenous catalysts eventually.
Ammonia is normally talked about to have further hydrogen than molecular hydrogen, explains Jesum Alves Fernandes from the School of Nottingham throughout the UK. This extreme hydrogen content material materials and its ease of liquefaction indicate many evaluation groups are exploring ammonia’s potential as a carbon-neutral gasoline and what provides perform biggest to catalyse its decomposition into nitrogen and hydrogen.
Diversified metal-based catalysts have been developed for ammonia decomposition and catalysts primarily based totally on ruthenium seem like among the many many solely. Now, Alves Fernandes and colleagues have demonstrated how a ruthenium catalyst they’ve developed turns into further full of life with time and the single-particle changes that account for its effectivity.
The workforce prepared their catalyst using magnetron sputtering. ‘We realised that if we are going to deal with the ground diffusion of metal atoms by controlling the obstacles to atom movement, along with components like temperature, density and the character of flooring defects, we are going to design catalytic centres of desired dimension and type,’ explains Alves Fernandes.

‘We do not use any solvents, reagents, ligands or totally different substances which can intervene [with any catalytic process]. In consequence, our methodology is extraordinarily controllable, reproducible and even programmable. Furthermore, no waste is generated, making this course of preferrred for large-scale fabrication,’ says Nottingham workforce member Andrei Khlobystov.
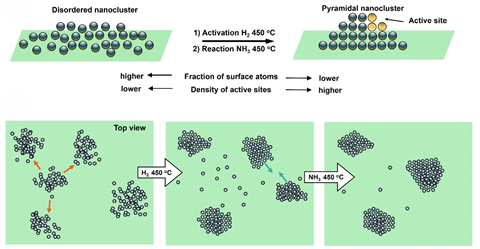
They then used comparable location-scanning transmission electron microscopy to hint the evolution of the particular person ruthenium nanoclusters all through a 20-hour-long ammonia decomposition response. ‘Each nanocluster undergoes interior changes, transitioning from amorphous shapes to crystalline nanoscale pyramids. These processes allow the catalyst to adapt to the response conditions and enhance its train,’ says Khlobystov. The following pyramids have an enormous proportion of five-atom ruthenium clusters, known as B5-site-rich ruthenium nanoparticles, which might be the full of life species in ammonia decomposition on ruthenium.
‘[The fact that the researchers] deal with to watch the full of life web sites at this scale, and the changes that improve its train, is unbelievable. The catalysts are typically dynamic and understanding what is going on on at this diploma is important,’ suggestions Laura Torrente Murciano, a specialist in ammonia cracking and synthesis on the School of Cambridge throughout the UK. ‘No matter starting with a disordered ruthenium layer that may very well be a poor catalyst, when the material is subjected to temperatures of 450°C beneath response conditions, reordering of the metal atoms happens, with an increase in train.’
Khlobystov signifies that every the fabrication strategy and the imaging strategy they use might presumably be utilized further extensively: ‘The imaging requires no additional specialist instruments except for the transmission electron microscope, which most institutes have’ Torrente Murciano moreover says ‘totally different comparable catalysts shall be prepared using their strategy – completely totally different areas of catalysis can all revenue.’